Title
Documents
Background paper on rapid COVID-19 testing
Polymerase chain reaction (PCR) and Reverse transcription-polymerase chain reaction (RT-PCR) are widely considered the ‘gold standard’ for medical diagnostics. However, PCR and RT-PCR testing requires a fully functioning lab, a strong supply chain, long wait times, and trained technicians, which may be lacking in some resource-constrained testing locations. Rapid testing has been studied as a quicker and easier diagnostic method for low-resource settings, especially for diseases with high transmission rates such as COVID-19. Tracking of infected individuals for early detection using Information & Communication Technologies (ICT) solutions, and lung ultrasound are increasingly used in resource-constrained settings
Background paper on innovations in medical devices and PPEs
During the COVID-19 pandemic there have been worldwide shortages of critical personal protective equipment (PPE) and medical devices, primarily ventilators and oxygen therapy devices. In response to the increased demand for these technologies during the pandemic, the U.S FDA enacted Emergency Use Authorizations (EUA) during the COVID-19 crisis to expand the availability of PPE, ventilators, and other critical medical equipment. EUAs intend to increase the availability of PPEs and medical devices to respond to the COVID-19 crisis by allowing flexibility for manufacturers to make appropriate modifications to the design of these devices to address current supply shortages. These special authorizations have led to the development of innovative design and manufacturing approaches for PPE and ventilator technologies. Although the increased need for these technologies has sparked the development of open source and locally and rapidly manufactured solutions, there are many design and manufacturing standards that must be followed to uphold quality.
https://www.uneca.org/sites/default/files/images/Science_Tech/background_paper_ppe_6.10.pdf
Following the successful completion of the Africa Innovation and Investment Forum 2020 took place 15– 19 June 2020; 7 outstanding and successful innovations and products have been selected from the total of 168 submissions.
Winners of the First Africa Innovation and Investment Forum 2020; Innovation Challenge and Innovation in Government Challenge.
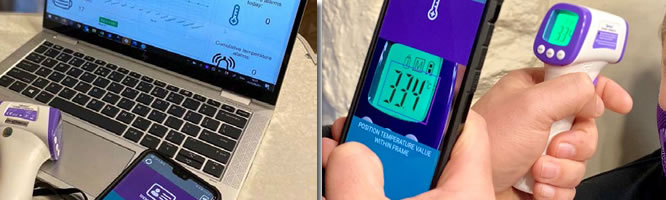
***Winner*** Investment Ready Rapid and Point-of Care Testing Innovation
Mountaga keita, Republic of Guinea
Email: mountagak@tulipindustry.com Focus Area: Medical Technologies Preject Key Word: Telemedicine Kiosks / Covid-19 Symptoms Scanner Tablets
| |
***Winners*** Investment Ready Medical Devices and Personal Protection Equipment
Shona McDonald , South Afirca
Email: shona@shonaquip.co.za Focus Area: ventilator and PPE Preject Key Word: Respirators, Local Production, Business for Good, Inclusive Local Employment.
Yared Yaregal, Ethiopia Email: yared.yaregal@hahucomputers.com Focus Area: Multi Patient Ventilation System and Patient Mentoring Preject Key Word: Medical Device, BioTech, Bio Medical Engineering
| |
*** Winner*** Investment Ready Personal Protection Equipment Technologies and System
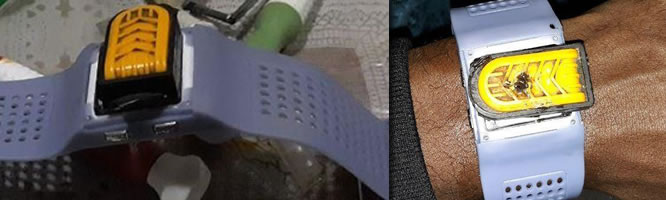
Tewodros Tessema, Ethiopia
Email: tayalew950@gmail.com Focus Area: Portable Sanitizer delivery tool with wristband Preject Key Word: Sanitizer delivery tool
| |
| |
| |
***Winner**** investment ready innovation in government
Corrin Varady, South Africa
Email: corrin@myideafrica.com Focus Area: Digital curriculum, assessment, professional development and data analytics, Learner Management System and digital content Preject Key Word: Digital Education, Interactive Learning, Virtual Schools, Professional Development, Digital Transformation, 21st Century Learning, Remote Learning
|